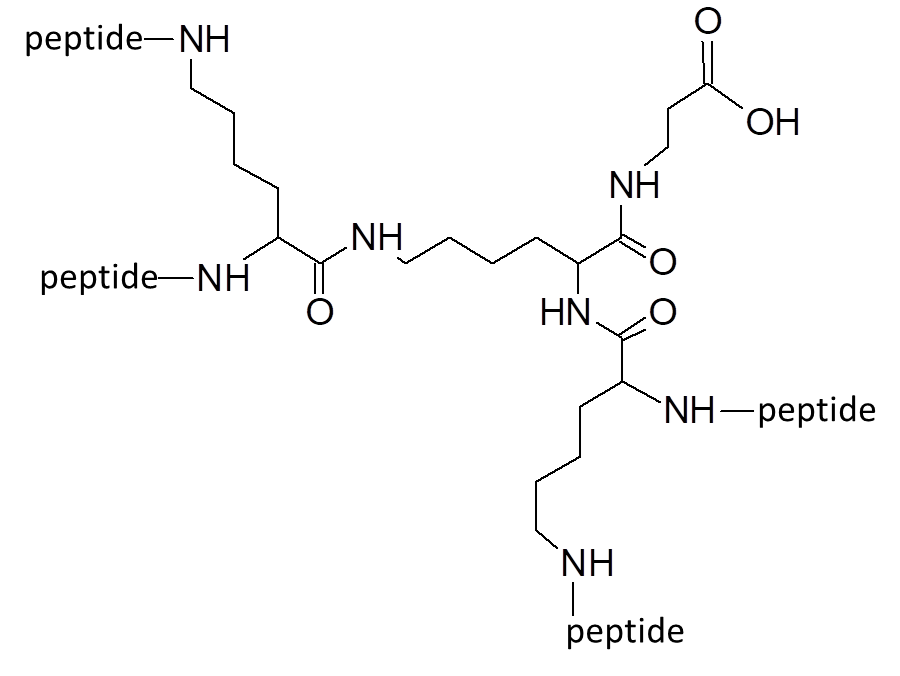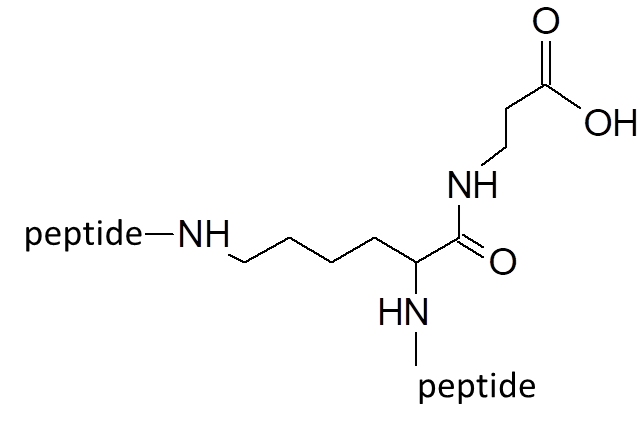SetLance srl
SetLance exploits a tetra-branched or di-branched peptide format for the construction of long-lived molecules to be developed as new drugs.
Setlance technology is based on the design, synthesis and efficacy evaluation of new bioactive peptides.
Our most bioactive peptide molecules are branched: multimericity determines increased protease stability and avidity of binding.
Setlance has now well established experience in the field of antimicrobial peptides and cancer-targeting peptides.
The proprietary antimicrobial peptide family has been scanned for in vitro and in vivo efficacy, toxicity in cells and animals, antibiofilm activity, antiinflammatory activity and efficacy when incorporated in nanosystems.
Tumor targeting peptides, conjugated to chemotherapeutics resulted promising in in vitro and in vivo cancer killing, either in the free form and incorporated in nanosystems.
SetLance develops new lead peptide sequences, which are optimized, characterized in vitro, and eventually developed in the preclinical, and early clinical phases.
Our most bioactive peptide molecules are branched: multimericity determines increased protease stability and avidity of binding.
Setlance has now well established experience in the field of antimicrobial peptides and cancer-targeting peptides.
The proprietary antimicrobial peptide family has been scanned for in vitro and in vivo efficacy, toxicity in cells and animals, antibiofilm activity, antiinflammatory activity and efficacy when incorporated in nanosystems.
Tumor targeting peptides, conjugated to chemotherapeutics resulted promising in in vitro and in vivo cancer killing, either in the free form and incorporated in nanosystems.
SetLance develops new lead peptide sequences, which are optimized, characterized in vitro, and eventually developed in the preclinical, and early clinical phases.
References
Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high density multiple antigenic peptide system. Proc.Natl.Acad.Sci.USA. 1988, 85, 5409–5413
Bracci L, Falciani C, Lelli B, Lozzi L, Runci Y, Pini A, De Montis MG, Tagliamonte A, Neri P. Synthetic peptides in the form of dendrimers become resistant to protease activity. J Biol Chem. 2003, 278:46590-5.
Falciani C, Lozzi L, Pini A, Bracci L. Bioactive peptides from libraries. Chem Biol. 2005, 12:417-26.
Falciani C, Lozzi L, Pini A, et al. Molecular basis of resistance to enzyme proteolysis. Chem Biol Drug Des. 2007, 69:216–21.
Pini A, Falciani C, Bracci L. Branched peptides as therapeutics. Curr Protein Pept Sci. 2008, 9:468-77